In an underground facility beneath Duke's campus – the Triangle Universities Nuclear Laboratory (TUNL) – researchers are testing a new approach to radiation therapy that could transform how brain tumors are treated. Termed FLASH radiation therapy, this technique delivers the same total dose of radiation as traditional radiation therapy, but in ultra-fast bursts.
A recent Duke-led study published in October in the International Journal of Radiation Oncology, Biology, Physics, "Investigating the FLASH Effect in a Rat Brain Organotypic Model with a Novel High Energy Electron Beam," showcases how researchers are using this technology to better understand FLASH's effects on both cancer and the brain's immune system – potentially paving the way for safer and more effective radiation treatment.

Study authors used the TUNL's high intensity gamma-ray source (HIGS) – the highest-flux Compton gamma-ray source in the world – at the Duke Free Electron Laser Laboratory to create a custom-built beamline capable of delivering this ultra-high dose rate radiation to brain tissue samples paired with metastatic breast cancer cells, mimicking the conditions of brain metastases.
FLASH research work at Duke started in 2021 when researchers in the Medical Physics Graduate Program reached out to Ying Wu, PhD, in the Duke Department of Physics.
"Professor Wu oversees the HIGS beam line, and with limited resources generously managed to work some magic to enable the first FLASH experiments squeezed in between other nuclear physics research teams from around the world. These experiments confirmed not only proof of feasibility but also the astonishing capability of HIGS-FLASH – capable of dose rates in excess of 20 million Gy/s for doses up to 20 Gy with 35 or 180MeV electrons," said Mark Oldham, PhD, physics lead for FLASH work at Duke. "We're particularly grateful for the dedication and efforts of several Medical Physics graduate students to date – Markus Sprenger, Tyler Kay and Victoria Parker – who have all made significant contributions and successfully defended their theses on the FLASH projects."
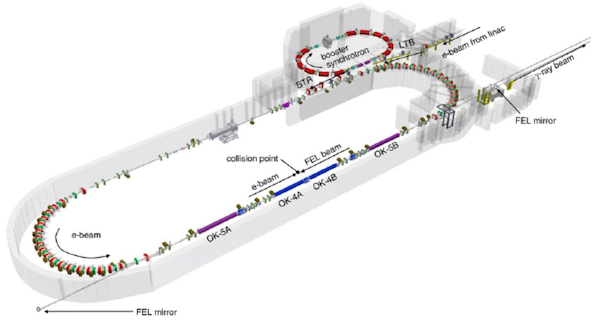
"After we approached our TUNL colleagues, as conversations evolved, we realized we might be able to deliver one of the fastest FLASH beams available using their technology," said study author and radiation oncologist Zach Reitman, MD, PhD. "It's a unique setup – a massive underground particle accelerator originally built for astrophysics experiments. Over the past few years, we've done countless hours of experiments that have proven that yes, this beam can deliver FLASH – and in fact, it has one of the highest intrapulse dose rates."
The beam allows researchers to run sophisticated preclinical experiments and ask critical questions about how FLASH works – and how to make it work better. For example, they’re exploring how the intra-pulse dose rate, or the amount of radiation that is delivered during a single burst, might affect biological outcomes. “We’re essentially turning the knobs on this machine to figure out what makes FLASH effective,” said Dr. Reitman. “Is it the speed? The dose? The spacing of the pulses? These are things we can now study in detail at Duke.”
“We are extremely excited that the HIGS-FLASH beam line shows such promise, and we think this type of technology could revolutionize radiation therapy,” said Scott Floyd, MD, PhD, one of the FLASH researchers at Duke, who credits the project’s early success to their first grant received – a spring 2023 Duke Cancer Institute pilot award. “The support provided by the Duke Cancer Institute propelled the project forward, and allowed us to generate critical data for publications and subsequent grants to continue the project.”
As research progresses, scientists are uncovering even more intriguing aspects of FLASH. One unique feature is its apparent ability to spare healthy tissue while still effectively targeting tumor cells – a phenomenon that is still not fully understood.
The study team’s findings add an important piece to that puzzle. They found that although cancer growth was similarly slowed by both FLASH and conventional radiation therapy, the FLASH-treated brain tissue showed distinct differences in immune signaling. In particular, the researchers found elevated levels of TNFα and fractalkine – molecules that play a role in inflammation – and altered behavior in immune cells of the brain. These changes suggest that FLASH doesn’t just reduce collateral tissue damage – it may actually change how the immune system interacts with tumors.
These immune effects take on even greater significance in light of another study published in Nature Cancer in March; the study is co-authored by Dr. Reitman, conducted in collaboration with researchers at the University of Pennsylvania and titled “FLASH radiation reprograms lipid metabolism and macrophage immunity and sensitizes medulloblastoma to CAR-T cell therapy.” Findings showed that FLASH appeared to enhance the effectiveness of CAR-T cell therapy, a form of immunotherapy that has had limited success in treating solid tumors like brain cancers. “CAR-T cells have been revolutionary for blood cancers, but they haven’t worked well in the brain,” said Dr. Reitman. “What this work suggests is that FLASH may help change that – it may reprogram the tumor environment to be more receptive to immune attack.”
This potential is also exciting in the context of pediatric brain tumors, where treatment options are limited and long-term side effects from traditional radiation therapy can be devastating. In the editorial “FLASH Radiation: A Game-Changer in Pediatric Brain Tumor Treatment?” published in the International Journal of Radiation Oncology, Biology, Physics in November, Dr. Reitman and his co-author explore the promise of FLASH for this population. “Children’s brains are still developing, and radiation can cause lifelong neurocognitive and physical problems,” he said. “If FLASH can preserve healthy tissue better, it could fundamentally change the way we treat kids with brain tumors.”
Although FLASH is not yet ready for patients – the technology is still experimental and not approved for clinical use – the future looks promising. Duke’s $150 million proton therapy center is slated to open in 2029, and clinical trials at other institutions are already underway for certain cancers. “There’s a world in which FLASH becomes the standard for many types of radiation therapy,” said Dr. Reitman. “But for now, we’re doing the work to understand it – to make sure that when the time comes, we’ll be ready.”
For those interested in collaboration, please contact Mark Oldham, PhD.