Lee Lab Overview

The overall goal of the Lee Lab is to improve the therapeutic window of radiation therapy and the survivorship of cancer patients by minimizing the acute and late effects of radiation. The lab studies mechanisms underlying radiation-induced tissue injury and regeneration to develop novel medical countermeasures and predictive biomarkers. The Lee Lab is supported by active NIH grants studying radiation-induced oral mucositis (R01DE033404), gastrointestinal acute radiation syndrome (U01AI186969 and R21AI193496), radiation-induced intestinal fibrosis (U01AI183940) and radiation-induced heart disease (U01AI189426).
Current Research Interests
My lab utilizes complementary approaches, including genetically engineered mouse models, single-cell sequencing and organoids, to study:
- Critical signaling pathways that regulate the reprogramming and regeneration of intestinal and oral epithelium following acute radiation injury
- Mechanisms of radiation-induced fibrosis in the intestines and the heart
By understanding the fundamental mechanisms, we aim to develop novel medical countermeasures and predictive biomarkers for radiation-induced normal tissue injury.
Ongoing Projects
- Radioprotective effect of p53 against oral mucositis (R01DE033404)
- Mitigation of gastrointestinal acute radiation syndrome by promoting clusterin-mediated intestinal regeneration (U01AI186969)
- Epigenetic mechanisms of intestinal stem cell fate conversion in response to severe radiation injury (collaboration with Dr. Christine Eyler) (R21AI193496)
- Sex differences in delayed development of gastrointestinal fibrosis after acute radiation exposure (collaboration with Dr. Jatin Roper) (U01AI183940)
- Serum pro-N-cadherin as an early biomarker of radiation-induced heart disease (collaboration with Drs. Salvatore Pizzo, Manisha Palta, and Christopher Kelsey) (U01AI189426)
Research
Mechanisms underlying the development of radiation-induced oral mucositis
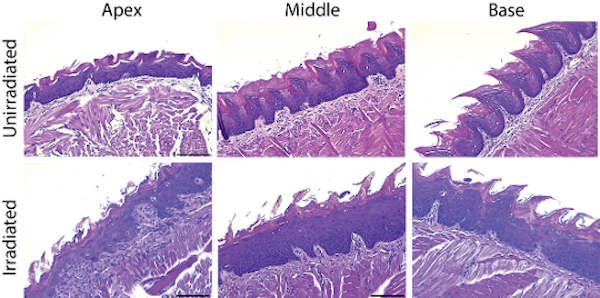
Head and neck squamous cell carcinoma (HNSCC) represents the sixth most common cancer worldwide. The Global Cancer Observatory predicts a 30% increase in annual incidence by 2030 with approximately 1.08 million new cases/year. Radiation therapy (RT) plays an integral role in treating HNSCC; however, head and neck RT is associated with significant toxicity in the oral mucosa. This toxicity, termed radiation-induced oral mucositis (RIOM), can lead to opioid use, reduced oral intake/poor nutritional status and the need for treatment breaks, all of which are correlated with worse outcomes for patients with HNSCC. As current treatment options for RIOM are limited, there is an unmet need to develop novel radioprotectors that will widen the therapeutic window of head and neck RT. Our long-term goal aims to develop novel therapeutic strategies that will prevent or reduce RIOM without sacrificing tumor control.
Mechanisms underlying the regeneration and reprogramming of intestinal epithelial cells in response to severe radiation injury
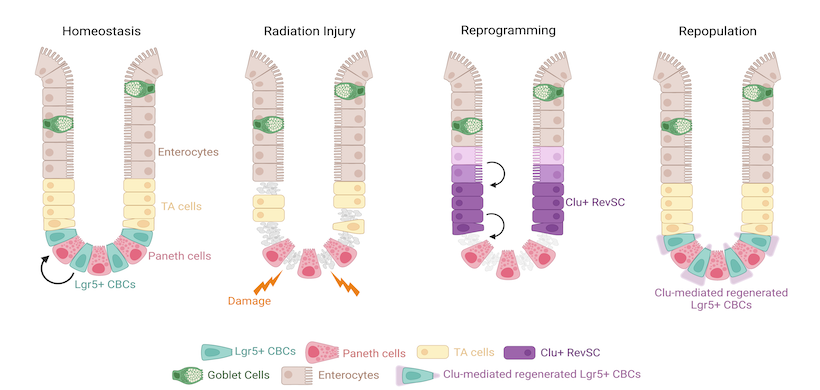
Radiation tolerance of the gastrointestinal (GI) tract can be exceeded in radiation accidents at nuclear power plants or after the detonation of a nuclear weapon or radioactive bomb, resulting in a lethal GI acute radiation syndrome (GI-ARS). There are currently no FDA-approved medical countermeasures that can mitigate GI-ARS. To develop novel approaches that ameliorate this life-threatening consequence of radiation exposure, there is a critical need to identify key signaling nodes that control the regeneration of intestinal epithelial cells in response to lethal radiation injury. The long-term goal of this project is to develop effective medical countermeasures against GI-ARS by gaining deeper insights into the mechanisms of intestinal regeneration after radiation injury.
Publications
Featured Publications
Normal tissue injury from radiation exposure
- Lee CL, Moding EJ, Cuneo KC, Li Y, Sullivan JM, Mao L, Washington I, Jeffords LB, Rodrigues RC, Ma Y, Das S, Kontos CD, Kim Y, Rockman HA, Kirsch DG. p53 Functions in Endothelial Cells to Prevent Radiation-Induced Myocardial Injury in Mice. Science Signaling. 2012 Jul 24; 5(234). PMCID: PMC3533440.
- Lee CL, Min H, Befera N, Clark D, Qi Y, Das S, Johnson GA, Badea CT, Kirsch DG. Assessing Cardiac Injury in Mice with Dual Energy-microCT, 4D-microCT, and microSPECT Imaging After Partial Heart Irradiation. International Journal of Radiation Oncology, Biology, Physics. 2014 Mar 1;88(3):686-693. PMCID: PMC3985387.
- Lee CL*, Lento WE*, Castle KD, Chao NJ, Kirsch DG. Inhibiting Glycogen Synthase Kinase-3 Mitigates the Hematopoietic Acute Radiation Syndrome in Mice. Radiation Research. 2014 May; 181(5):445-451. PMCID: PMC4080719. (*Equal contribution)
- Lee CL, Oh P, Xu E, Ma Y, Kim Y, Daniel AR, Kirsch DG. Blocking cyclin-dependent kinase 4/6 during single dose vs. fractionated radiation therapy leads to opposite effects on acute gastrointestinal toxicity in mice. International Journal of Radiation Oncology, Biology, Physics. 2018 Jul 26. pii: S0360-3016(18)31645-6. doi: 10.1016/j.ijrobp.2018.07.192. [Epub ahead of print] PubMed PMID: 30056081.
- Lee CL, Daniel AR, Holbrook M, Brownstein J, Silva Campos LD, Hasapis S, Ma Y, Borst LB, Badea CT, Kirsch DG. Sensitization of Vascular Endothelial Cells to Ionizing Radiation Promotes the Development of Delayed Intestinal Injury in Mice. Radiation Research. 2019 Sep;192(3):258-266. doi: 10.1667/RR15371.1. Epub 2019 Jul 2. PubMed PMID: 31265788.
- Lee CL*, Lee JW*, Daniel AR, Holbrook M, Hasapis S, Wright AO, Brownstein J, Da Silva Campos L, Ma Y, Mao L, Abraham D, Badea CT, Kirsch DG. Characterization of cardiovascular injury in mice following partial-heart irradiation with clinically relevant dose and fractionation. Radiotherapy and Oncology. 2021 Feb 2:S0167-8140(21)00025-6. doi: 10.1016/j.radonc.2021.01.023. Epub ahead of print. PMID: 33545252. (*Equal contribution)
- Sheahan BJ, Freeman AN, Keeley TM, Samuelson LC, Roper J, Hasapis S, Lee CL, Dekaney CM. Epithelial regeneration after doxorubicin arises primarily from early progeny of active intestinal stem cells. Cellular Molecular Gastroenterology Hepatology. 2021 Feb 8:S2352-345X(21)00021-7. doi: 10.1016/j.jcmgh.2021.01.015. Epub ahead of print. PMID: 33571711.
- Lee CL, Wright AO, Lee JW, Brownstein J, Hasapis S, Satow S, Da Silva Campos L, Williams N, Ma Y, Luo L, Johnson T, Daniel AR, Harrison WT, Oldham M and Kirsch DG. Sensitization of endothelial cells to ionizing radiation exacerbates delayed radiation myelopathy in mice. Radiation Research. 2021 Nov 1;197(3):0. doi:10.1667/RADE-21-00166.1. PMID: 34724704; PMCID: PMC8567311.
- Morral C, Ayyaz A, Kuo HC, Fink M, Verginadis I, Daniel AR, Burner DN, Driver LM, Satow S, Hasapis S, Ghinnagow R, Luo L, Ma Y, Attardi LD, Koumenis C, Minn AJ, Wrana JL*, Lee CL*, Kirsch DG*. p53 promotes revival stem cells in the regenerating intestine after severe radiation injury. Nature Communications. 2024 Apr 8;15(1):3018. doi: 10.1038/s41467-024-47124-8. PMID: 38589357; PMCID: PMC11001929. (*Co-corresponding authors)
Radiation-induced carcinogenesis
- Lee CL, Castle KD, Moding EJ, Blum JM, Williams N, Luo L, Ma Y, Borst L, Kim Y, Kirsch DG. Acute DNA Damage Activates the Tumor Suppressor p53 to Promote Radiation-induced Lymphoma. Nature Communications. 2015 Sep 24;6:8477. PMCID: PMC4586051.
- Moding EJ, Min HD, Castle KD, Ali M, Woodlief L, Williams N, Ma Y, Kim Y, Lee CL, Kirsch DG. An Extra Copy of p53 Suppresses Development of Spontaneous Kras-driven but not Radiation-Induced Cancer. JCI Insight. 2016 Jul 7;1(10). pii:e86698. PMCID:PMC4955525.
- Lee CL*, Mowery YM*, Daniel AR*, Zhang D, Sibley AB, Delaney JR, Wisdom AJ, Qin X, Wang X, Caraballo I, Gresham J, Luo L, Van Mater D, Owzar K, Kirsch DG. Mutational landscape in genetically engineered, carcinogen-induced, and radiation-induced mouse sarcoma. JCI Insight. 2019 May 21;5. pii: 128698. doi: 10.1172/jci.insight.128698. PubMed PMID: 31112524. (*Equal contribution)
- Hasapis S, Caraballo I, Lee CL. Transplantation of unirradiated bone marrow cells following total-body irradiation prevents the development of thymic lymphoma in mice through niche competition. Radiation Research. 2021 Mar 1;195(3):301-306. doi:10.1667/RADE-20-00221.1. PMID: 33347573; PMCID: PMC8240018.
- Lee CL*, Brock KD, Hasapis S, Zhang D, Sibley AB, Qin X, Gresham JS, Caraballo I, Luo L, Daniel AR, Hilton MJ, Owzar K, and Kirsch DG*. Whole-exome sequencing of radiation-induced thymic lymphoma in mouse models identifies Notch1 activation as a driver of p53 wild-type lymphoma. Cancer Research 2021 May 25:canres.2823.2020.doi: 10.1158/0008-5472.CAN-20-2823. Epub ahead of print. PMID: 34035082. (*Co-corresponding authors)
- Daniel AR, Su C, Williams NT, Li Z, Huang J, Lopez O, Luo L, Ma Y, Campos LDS, Selitsky SR, Modliszewski JL, Liu S, Hernansaiz-Ballesteros R, Mowery YM, Cardona DM, Lee CL, Kirsch DG. Transient knockdown of p53 during focal limb irradiation increases the development of sarcomas. Cancer Research Communications. 2023 Nov 2. doi: 10.1158/2767-9764.CRC-23-0104. Epub ahead of print. PMID: 37982576.
Tumor responses to radiation therapy
- Lee CL*, Moding EJ*, Huang X, Li Y, Woodlief LZ, Rodrigues RC, Ma Y, Kirsch DG. Generation of Primary Tumors with Flp Recombinase in FRT-flanked p53 Mice. Disease, Models, and Mechanisms. 2012 May; 5(3):397-402. PMCID: PMC3339833. (*Equal contribution)
- Moding EJ, Lee CL, Castle KD, Oh P, Mao L, Zha S, Min HD, Ma Y, Das S, Kirsch DG. ATM Deletion with Dual Recombinase Technology Preferentially Radiosensitizes Tumor Endothelium. Journal of Clinical Investigation. 2014 Aug 1; 124(8):3325-38. PMCID: PMC4109553.
- Moding EJ, Castle KD, Perez BA, Oh P, Min HD, Norris H, Ma Y, Cardona DM, Lee CL, Kirsch DG. Tumor Cells, but not Endothelial Cells, Mediate Eradication of Primary Sarcomas by Stereotactic Body Radiation Therapy. Science Translational Medicine. 2015 Mar 11;7(278): 278ra34. PMCID: PMC4360135.
- Torok JA, Oh P, Castle KD, Reinsvold M, Ma Y, Luo L, Kuo HC, Lee CL, Kirsch DG. Atm deletion in tumor but not endothelial cells improves radiation response in a primary mouse model of lung adenocarcinoma. Cancer Research. 2018 Oct 12. pii: canres.3103.2017. doi: 10.1158/0008-5472.CAN-17-3103. [Epub ahead of print] PubMed PMID: 30315114.
People
Lee Lab – Alumni
- Ayra Charania
Undergraduate Student
- Paul Kim
Undergraduate Student
- Sloane Satow
Undergraduate Student
- John Cart, MS
Research Technician II
- Davis Brock, BS
Research Technician II
- Isibel Caraballo, BS
Research Technician II
- Lucas Norris
Research Technician II
- Stephanie Hasapis, BS, LAT
Lab Research Analyst I
- Jessica Lee, MD
Resident, Radiation Oncology
- Reynier David Rodriguez Rosales
OPSD PRIME Academy 2022
- Abdul-Rahman Saleh
OPSD PRIME Academy 2021
News
Lee, Palta, Pizzo Awarded $2.8 Million U01 for Research on Radiation-Induced Heart Disease
Lee, Eyler Awarded R21 for Research on GI-ARS
Lee Awarded U01 for Research on Severe Radiation Injury
Lee Lab Receives U01 to Study Sex Differences in Radiation-Induced Intestinal Fibrosis
Lee Receives R01 for Research on Radiation-Induced Oral Mucositis
Contact
Visit Dr. Lee's Scholars@Duke profile. Contact Dr. Lee at chang-lung.lee@duke.edu.










