Reitman Lab Overview
The goal of the Reitman Lab is to make discoveries that guide the design of improved treatment strategies for children and adults with brain tumors. The Reitman Lab is based in the Department of Radiation Oncology and the team works closely with the faculty and staff in the Preston Robert Tisch Brain Tumor Center at Duke, the Department of Neurosurgery and the Duke Cancer Institute.

Reitman Lab members in winter 2023

Reitman Lab members in winter 2022

Reitman Lab members in June 2022 in front of Duke Chapel
Research
The goal of the Reitman Lab is to make discoveries that guide the design of improved treatment strategies for children and adults with brain tumors. We leverage molecular biology techniques, genetically engineered mouse models and cancer genomic approaches to carry out our work. The Reitman Lab is based in the Department of Radiation Oncology and the team works closely with the faculty and staff in the Preston Robert Tisch Brain Tumor Center at Duke, the Department of Neurosurgery and the Duke Cancer Institute. You can find Dr. Reitman's NIH Biosketch here and academic CV here.

We are interested in several broad research themes:
Enhancing the efficacy of radiation therapy
Radiation therapy plays a critical role in the treatment of many brain tumor patients. For many patients with brain tumors, radiation therapy is part of a curative treatment regimen but can be associated with long-term toxicity. For patients with other types of brain tumors, toxicity to normal tissues limits the ability to deliver a curative dose of radiation therapy to the brain tumor. The Reitman Lab is investigating new approaches to widen the therapeutic ratio of radiation therapy by making it more effective in killing brain tumor cells and helping to reduce toxicity to normal tissues. For example, one approach involves studying the DNA damage response molecular pathway that regulates the cellular response to radiation. Genes involved in the DNA damage response pathway are mutated in many brain tumors, especially in gliomas of the brainstem (Figure 1). Since these tumors have deregulated DNA damage response machinery, treatments that target key nodes in the DNA damage response network might be particularly effective in sensitizing these tumors to radiation therapy. We are using primary genetically engineered mouse models of diffuse midline gliomas to test if modulating key DNA damage response molecules can sensitize these tumors to radiation therapy.
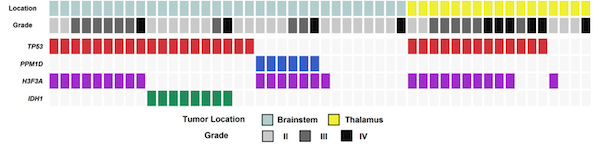
Figure 1. Frequent DNA damage response pathway gene mutations in brainstem and thalamus gliomas.
Each column represents a patient with a brainstem or thalamus glioma. This includes predominantly diffuse midline gliomas with H3K27M mutations (H3F3A mutated) and diffuse intrinsic pontine gliomas (DIPGs). The top two rows delineate the location and histopathologic grade of the tumor. The bottom four rows indicate the presence of mutations in key cancer genes in each tumor specimen. The data show that the >50% of these brain tumors harbor inactivating mutations in the tumor suppressor TP53, which regulates apoptosis and cell cycle progression especially after DNA damage is caused by radiation therapy. Additional tumors that do not contain TP53 mutations instead contain mutations that activate PPM1D, which encodes a phosphatase that dephosphorylates and represses p53 function. Thus, the majority of these brain tumors contain mutations that deregulate the DNA damage response pathway by perturbing p53 function. We are testing if targeting the serine/threonine kinase ataxia telangiectasia mutated (ATM), which plays a critical role in the detection of DNA damage caused by radiation therapy, can specifically radiosensitize diffuse midline gliomas with TP53 or PPM1D mutations. This work is primarily being carried out in genetically engineered mouse models of diffuse midline gliomas that faithfully replicate the genetic mutations in these tumors.
Figure from: Zhang L… Reitman ZJ, Bigner DD, Yan H. Exome sequencing identifies somatic gain-of-function PPM1D mutations in brainstem gliomas. Nat Genet. 2014 PMID: 24880341
New approaches to target brain tumor mutations
Knowledge of the genomic landscape of brain tumors has exploded over the past decade. In many types of cancer outside the brain, this type of genomic knowledge has resulted in new therapies that improve patient survival by targeting mutations found in the genome of the cancer cells. However, advances in genomic characterization of brain tumors have not yet produced new survival-improving therapies for many types of brain tumors. To overcome this challenge, the Reitman Lab is investigating creative new approaches to target frequent mutations found in brain tumors. For instance, we are interested in finding novel actionable vulnerabilities associated with mutations in the promoter of the telomerase reverse transcriptase gene (TERT). TERT promoter mutations are found in more than 80% of glioblastomas, which are the most frequent and lethal primary brain tumor in adults, and the mutations confer a replicative immortality phenotype to brain tumor cells (Figure 2).
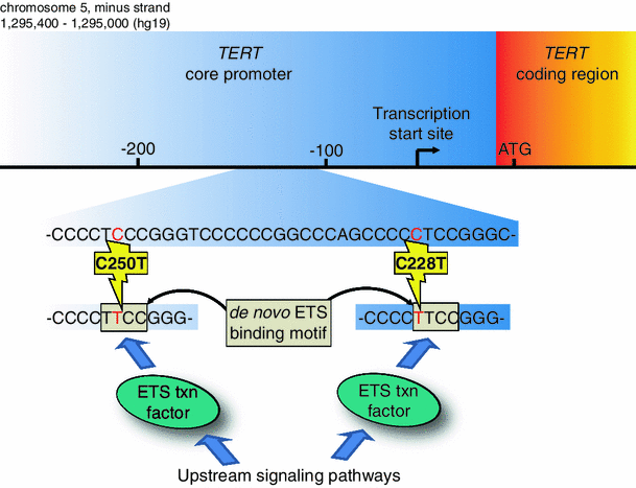
Figure 2. Identifying new approaches to target TERT promoter mutations in cancer.
The two most frequent TERT promoter mutations found in glioblastoma are shown. The C228T mutation occurs 146 bp upstream of the start codon of TERT. The C250T mutation occurs 126 bp upstream of the start codon of TERT. Either mutation generates a de novo sequence which contains an ETS transcription factor binding motif. The new ETS binding motif aberrantly recruits transcription factors from the ETS family to the TERT promoter. This aberrantly activates TERT expression to enable replicative immortality in glioblastoma cells. Our long-term goal is to determine if targeting molecular pathways upstream of these transcription factors could be used as a cancer therapeutic approach for tumors with TERT promoter mutations. To identify such vulnerabilities, we are using genome-wide CRISPR-based screening approaches in patient-derived glioblastoma cell lines faithfully harboring TERT promoter mutations.
Figure from: Reitman ZJ, et al. Promoting a new brain tumor mutation: TERT promoter mutations in CNS tumors. Acta Neuropathol. 2013 PMID: 24217890
Examining tumor heterogeneity and treatment resistance
Another hurdle to the success of new brain tumor treatments is that the tissue of many brain tumors is heterogeneous and does not uniformly respond to treatment. Furthermore, brain tumors evolve when the patient undergoes treatment, ultimately leading to treatment resistance. We are leveraging emerging technologies such as single cell RNA-sequencing to profile brain tumors at single cell resolution to define tumor heterogeneity and treatment resistance mechanisms in brain tumors. We are particularly interested in exploring pediatric brain tumors, low grade gliomas, glioneuronal tumors and tumors with mutations in the BRAF oncogene in this manner (Figure 3).
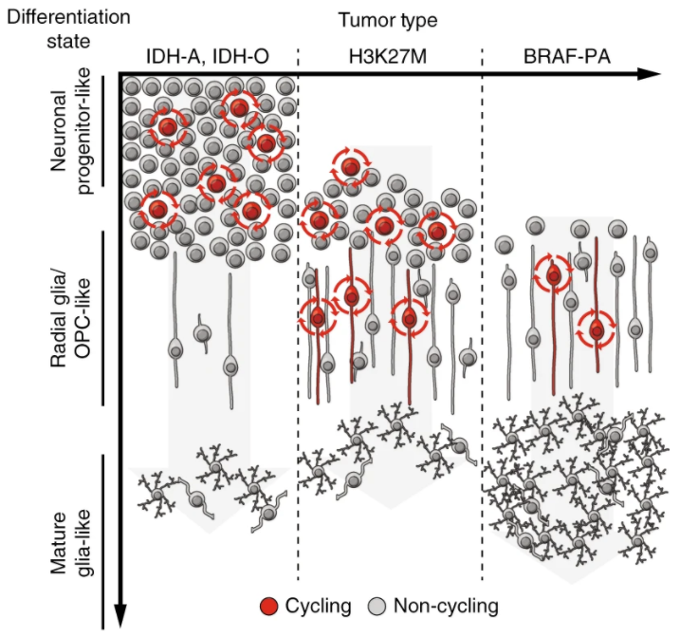
Figure 3. Single cell genomics to reveal brain tumor developmental hierarchies.
Model for glioma differentiation hierarchies based on single cell RNA-sequencing analyses of primary human brain tumors. The schematic shows differences in abundance of cycling cells and cellular differentiation state for various subtypes of gliomas. The y-axis shows differentiation state of the tumor cells, ranging from undifferentiated neuronal progenitor-like cells at the top to more differentiated mature glia-like cells at the bottom. The x-axis shows three major glioma subytpes, including IDH-mutated oligodendrogliomas (IDH-O) and astrocytomas (IDH-A), diffuse midline gliomas with H3K27M mutation and pilocytic astrocytomas with alterations in the BRAF oncogene (BRAF-PA). The BRAF-PAs resemble a more differentiated lineage hierarchy compared to the IDH- and H3K27M-mutated tumors. These findings may underlie the differing clinical behavior and varying responses to treatment of these brain tumor subtypes.
Figure from: Reitman ZJ, Paolella BR, et al., Mitogenic and progenitor gene programmes in single pilocytic astrocytoma cells. Nat Commun. 2019 PMID: 31427603
Other interests
We are also interested in innovatively harnessing gain-of-function cancer mutations to inform the design of valuable new enzymes, which could potentially be useful for drug and chemical production processes (Figure 4).
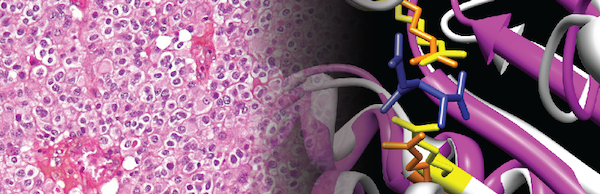
Figure 4. Enzyme redesign guided by cancer-derived mutations.
Cover artwork from Nature Chemical Biology showing (on the left) oligodendroglioma tumor cells, which contain an isocitrate dehydrogenase 1 (IDH1) R132H mutation. The active site of IDH1 is shown on the right. The R132H mutation affects a critical active site residue of IDH1, conferring a change-of-function to the IDH1 enzyme. We applied analogous mutations to the active sites of distantly related enzymes to redesign those enzymes into novel, useful enzymes with new functions. Future work seeks to identify additional cancer-derived mutations in enzyme active sites that could be used to guide the design of useful enzymes in a similar manner.
Figure from: Reitman ZJ, et al. Enzyme redesign guided by cancer-derived IDH1 mutations. Nat Chem Biol. 2012 PMID: 23001033
Additionally, we are interested in circulating tumor DNA (ctDNA) that is released from brain tumors into the circulation, especially as they undergo radiation therapy, to determine if testing for these byproducts could be used to improve the clinical management of brain tumor patients.
Support
The Reitman Lab has benefited from support from the Developmental Research Program and the Career Enhancement Program within the Duke Brain Tumor SPORE grant from the National Cancer Institute. The Lab has also received support from research foundations including the Pediatric Brain Tumor Foundation, the St. Baldrick’s Foundation, the Michael Mosier Defeat DIPG Foundation, the ChadTough Foundation, the SoSo Strong Foundation and the Conquer Cancer Foundation of the American Society for Clinical Oncology. Finally, support from the Duke Cancer Institute, the Preston Robert Tisch Brain Tumor Center at Duke and the Departments of Radiation Oncology and Neurosurgery are critical to carry out our work. We are extremely grateful to these sponsors and for the advocacy of patients and their families who make this research possible.
Lab Updates
November 2023
- The lab analyzes cancer genomic sequencing data to identify useful new enzymes, which may help to synthesize new drugs and other chemicals in the future. The work is entitled "Mining cancer genomes for change-of-metabolic-function mutations" in the Nature journal Communications Biology.
October 2023
- Kevin Tu and others in the lab analyze genetic screening data to uncover methods to target glioblastoma, one of the most deadly and aggressive types of brain tumors. The work is entitled "Pooled genetic screens to identify vulnerabilities in TERT-promoter-mutant glioblastoma" in Oncogene.
- Kevin Tu, an AMGEN scholar in the Reitman lab, begins a new chapter as a Churchill Scholar at the University of Cambridge, UK.
August 2023
- Harrison Liu delivered an oral presentation at the International Congress of Radiation Research entitled "Spatially-resolved transcriptomic analyses of radiosensitization strategies in a primary mouse model of diffuse midline glioma" in Montreal, Quebec, Canada.
- After two years in the lab, Loren Weidenhammer joins the Duke Pathology Department as a graduate student to pursue a PhD.
April 2023
- Sophie Wu is awarded an American Association for Cancer Research Undergraduate Scholar Award. She presented a poster entitled "Combining the RCAS/TVA retrovirus system and a conditional oncohistone H3.3K27M allele to investigate radiosensitization strategies in primary mouse models of diffuse midline glioma" at the American Association for Cancer Research Annual Meeting in Orlando, Florida.
March 2023
- Joshua Regal, MD, PhD, and others in the lab publish the first multi-omic analysis of ganglioglioma, an important brain tumor composed of cells that resemble neurons and glia. The work is entitled "Ganglioglioma deep transcriptomics reveals primitive neuroectoderm neural precursor-like population" in Acta Neuropathologica Communications.
- Loren Weidenhammer and others in the lab disseminate our unique protocols for brainstem glioma research in the Cell journal STAR Protocols.
November 2022
- Sophie Wu is awarded a Pratt Scholars award to continue her research on histone mutations and CRISPR/Cas9 techniques in mouse models of pediatric brain tumors
- Joshua Regal, MD, PhD, presents results on deep transcriptomic analysis of gangliogliomas and other glioneuronal brain tumors at Pediatric Oncology
October 2022
- Loren Weidenhammer presents results on roles of p53 tumor suppressor in our mouse models of pediatric brain tumors at the annual Radiation Research Society meeting in Hawaii
September 2022
- Connor Stewart and colleagues publish our work on a mouse model of brainstem glioma in Cancers
August 2022
- Kevin Tu completes a prestigious AMGEN undergraduate summer scholarship in the Reitman Lab
- Zach Reitman, MD, PhD, is awarded a K08256045 from the National Cancer Institute
July 2022
- Zach Reitman, MD, PhD, is awarded a third year of support from the Emily Beazley's Kures for Kids Fund to support the lab's research aimed at enhancing the efficacy of radiation therapy for childhood cancers
- Zach Reitman, MD, PhD, is awarded a New Investigator Award from ChadTough Defeat DIPG to study mechanisms of treatment resistance in childhood brain tumors
February 2022
- Maria Guerra Garcia and colleagues publish an important review on targeting Ataxia-telangiectasia mutated kinase (ATM) for future cancer treatments in Seminars in Radiation Oncology
Publications

-
A road map for the treatment of pediatric diffuse midline glioma. Koschmann C, Al-Holou WN, Alonso MM, Anastas J, Bandopadhayay P, Barron T, Becher O, Cartaxo R, Castro MG, Chung C, Clausen M, Dang D, Doherty R, Duchatel R, Dun M, Filbin M, Franson A, Galban S, Garcia Moure M, Garton H, Gowda P, Marques JG, Hawkins C, Heath A, Hulleman E, Ji S, Jones C, Kilburn L, Kline C, Koldobskiy MA, Lim D, Lowenstein PR, Lu QR, Lum J, Mack S, Magge S, Marini B, Martin D, Marupudi N, Messinger D, Mody R, Morgan M, Mota M, Muraszko K, Mueller S, Natarajan SK, Nazarian J, Niculcea M, Nuechterlein N, Okada H, Opipari V, Pai MP, Pal S, Peterson E, Phoenix T, Prensner JR, Pun M, Raju GP, Reitman ZJ, Resnick A, Rogawski D, Saratsis A, Sbergio SG, Souweidane M, Stafford JM, Tzaridis T, Venkataraman S, Vittorio O, Wadden J, Wahl D, Wechsler-Reya RJ, Yadav VN, Zhang X, Zhang Q, Venneti S. Cancer Cell. 2023 Nov 20:S1535-6108(23)00393-8. doi: 10.1016/j.ccell.2023.11.002. Online ahead of print. PMID: 38039965.
-
Single-fraction Radiation Treatment Dose Response in a Genetically Engineered Mouse Model of Medulloblastoma. Tu KJ, Stewart CE, Williams NT, Ma Y, Luo L, Ghosh D, Weidenhammer LB, Floyd SR, Fan Y, Kirsch DG, Oldham M, Reitman ZJ. Radiat Res. 2023 Nov 22. doi: 10.1667/RADE-23-00126.1. Online ahead of print. PMID: 37990957.
-
Mining cancer genomes for change-of-metabolic-function mutations. Tu KJ, Diplas BH, Regal JA, Waitkus MS, Pirozzi CJ, Reitman ZJ. Commun Biol. 2023 Nov 10;6(1):1143. doi: 10.1038/s42003-023-05475-w. PMID: 37950065.
-
Ataxia-telangiectasia mutated ( Atm ) disruption sensitizes spatially-directed H3.3K27M/TP53 diffuse midline gliomas to radiation therapy. Mangoli A, Wu S, Liu HQ, Aksu M, Jain V, Foreman BE, Regal JA, Weidenhammer LB, Stewart CE, Guerra Garcia ME, Hocke E, Abramson K, Williams NT, Luo L, Deland K, Attardi L, Abe K, Hashizume R, Ashley DM, Becher OJ, Kirsch DG, Gregory SG, Reitman ZJ. bioRxiv. 2023 Oct 20:2023.10.18.562892. doi: 10.1101/2023.10.18.562892. Preprint. PMID: 37904990.
-
ABL1 and ABL2 promote medulloblastoma leptomeningeal dissemination. Jones JK, Zhang H, Lyne AM, Cavalli FMG, Hassen WE, Stevenson K, Kornahrens R, Yang Y, Li S, Dell S, Reitman ZJ, Herndon JE 2nd, Hoj J, Pendergast AM, Thompson EM. Neurooncol Adv. 2023 Aug 7;5(1):vdad095. doi: 10.1093/noajnl/vdad095. eCollection 2023 Jan-Dec. PMID: 37781087.
-
Pooled genetic screens to identify vulnerabilities in TERT-promoter-mutant glioblastoma. Tu KJ, Stewart CE, Hendrickson PG, Regal JA, Kim SY, Ashley DM, Waitkus MS, Reitman ZJ. Oncogene. 2023 Oct;42(44):3274-3286. doi: 10.1038/s41388-023-02845-w. Epub 2023 Sep 23. PMID: 37741952.
-
Long-term outcomes with reduced-dose whole-brain radiotherapy and a stereotactic radiosurgery boost for primary central nervous system lymphoma. Foreman BE, Mullikin TC, Floyd SR, Kelsey CR, Patel MP, Peters KB, Kirkpatrick JP, Reitman ZJ, Vaios EJ. Neurooncol Adv. 2023 Aug 3;5(1):vdad097. doi: 10.1093/noajnl/vdad097. eCollection 2023 Jan-Dec. PMID: 37706200.
-
The ASNR-MICCAI Brain Tumor Segmentation (BraTS) Challenge 2023: Intracranial Meningioma. LaBella D, Adewole M, Alonso-Basanta M, Altes T, Anwar SM, Baid U, Bergquist T, Bhalerao R, Chen S, Chung V, Conte GM, Dako F, Eddy J, Ezhov I, Godfrey D, Hilal F, Familiar A, Farahani K, Iglesias JE, Jiang Z, Johanson E, Kazerooni AF, Kent C, Kirkpatrick J, Kofler F, Leemput KV, Li HB, Liu X, Mahtabfar A, McBurney-Lin S, McLean R, Meier Z, Moawad AW, Mongan J, Nedelec P, Pajot M, Piraud M, Rashid A, Reitman Z, Shinohara RT, Velichko Y, Wang C, Warman P, Wiggins W, Aboian M, Albrecht J, Anazodo U, Bakas S, Flanders A, Janas A, Khanna G, Linguraru MG, Menze B, Nada A, Rauschecker AM, Rudie J, Tahon NH, Villanueva-Meyer J, Wiestler B, Calabrese E. ArXiv. 2023 May 12:arXiv:2305.07642v1. Preprint. PMID: 37608937.
-
The Brain Tumor Segmentation (BraTS) Challenge 2023: Brain MR Image Synthesis for Tumor Segmentation (BraSyn). Li HB, Conte GM, Anwar SM, Kofler F, Ezhov I, van Leemput K, Piraud M, Diaz M, Cole B, Calabrese E, Rudie J, Meissen F, Adewole M, Janas A, Kazerooni AF, LaBella D, Moawad AW, Farahani K, Eddy J, Bergquist T, Chung V, Shinohara RT, Dako F, Wiggins W, Reitman Z, Wang C, Liu X, Jiang Z, Familiar A, Johanson E, Meier Z, Davatzikos C, Freymann J, Kirby J, Bilello M, Fathallah-Shaykh HM, Wiest R, Kirschke J, Colen RR, Kotrotsou A, Lamontagne P, Marcus D, Milchenko M, Nazeri A, Weber MA, Mahajan A, Mohan S, Mongan J, Hess C, Cha S, Villanueva-Meyer J, Colak E, Crivellaro P, Jakab A, Albrecht J, Anazodo U, Aboian M, Yu T, Chung V, Bergquist T, Eddy J, Albrecht J, Baid U, Bakas S, Linguraru MG, Menze B, Iglesias JE, Wiestler B. ArXiv. 2023 Jun 28:arXiv:2305.09011v5. Preprint. PMID: 37608932.
-
Clinical Applications of Immunotherapy for Recurrent Glioblastoma in Adults. Olivet MM, Brown MC, Reitman ZJ, Ashley DM, Grant GA, Yang Y, Markert JM. Cancers (Basel). 2023 Jul 31;15(15):3901. doi: 10.3390/cancers15153901. PMID: 37568717.
-
The Brain Tumor Segmentation (BraTS) Challenge 2023: Glioma Segmentation in Sub-Saharan Africa Patient Population (BraTS-Africa). Adewole M, Rudie JD, Gbdamosi A, Toyobo O, Raymond C, Zhang D, Omidiji O, Akinola R, Suwaid MA, Emegoakor A, Ojo N, Aguh K, Kalaiwo C, Babatunde G, Ogunleye A, Gbadamosi Y, Iorpagher K, Calabrese E, Aboian M, Linguraru M, Albrecht J, Wiestler B, Kofler F, Janas A, LaBella D, Kzerooni AF, Li HB, Iglesias JE, Farahani K, Eddy J, Bergquist T, Chung V, Shinohara RT, Wiggins W, Reitman Z, Wang C, Liu X, Jiang Z, Familiar A, Van Leemput K, Bukas C, Piraud M, Conte GM, Johansson E, Meier Z, Menze BH, Baid U, Bakas S, Dako F, Fatade A, Anazodo UC. ArXiv. 2023 May 30:arXiv:2305.19369v1. Preprint. PMID: 37396608.
-
The Brain Tumor Segmentation (BraTS-METS) Challenge 2023: Brain Metastasis Segmentation on Pre-treatment MRI. Moawad AW, Janas A, Baid U, Ramakrishnan D, Jekel L, Krantchev K, Moy H, Saluja R, Osenberg K, Wilms K, Kaur M, Avesta A, Pedersen GC, Maleki N, Salimi M, Merkaj S, von Reppert M, Tillmans N, Lost J, Bousabarah K, Holler W, Lin M, Westerhoff M, Maresca R, Link KE, Tahon NH, Marcus D, Sotiras A, LaMontagne P, Chakrabarty S, Teytelboym O, Youssef A, Nada A, Velichko YS, Gennaro N; Connectome Students; Group of Annotators; Cramer J, Johnson DR, Kwan BYM, Petrovic B, Patro SN, Wu L, So T, Thompson G, Kam A, Perez-Carrillo GG, Lall N; Group of Approvers; Albrecht J, Anazodo U, Lingaru MG, Menze BH, Wiestler B, Adewole M, Anwar SM, Labella D, Li HB, Iglesias JE, Farahani K, Eddy J, Bergquist T, Chung V, Shinohara RT, Dako F, Wiggins W, Reitman Z, Wang C, Liu X, Jiang Z, Van Leemput K, Piraud M, Ezhov I, Johanson E, Meier Z, Familiar A, Kazerooni AF, Kofler F, Calabrese E, Aneja S, Chiang V, Ikuta I, Shafique U, Memon F, Conte GM, Bakas S, Rudie J, Aboian M. ArXiv. 2023 Jun 1:arXiv:2306.00838v1. Preprint. PMID: 37396600.
-
The Brain Tumor Segmentation (BraTS) Challenge 2023: Focus on Pediatrics (CBTN-CONNECT-DIPGR-ASNR-MICCAI BraTS-PEDs). Kazerooni AF, Khalili N, Liu X, Haldar D, Jiang Z, Anwar SM, Albrecht J, Adewole M, Anazodo U, Anderson H, Bagheri S, Baid U, Bergquist T, Borja AJ, Calabrese E, Chung V, Conte GM, Dako F, Eddy J, Ezhov I, Familiar A, Farahani K, Haldar S, Iglesias JE, Janas A, Johansen E, Jones BV, Kofler F, LaBella D, Lai HA, Van Leemput K, Li HB, Maleki N, McAllister AS, Meier Z, Menze B, Moawad AW, Nandolia KK, Pavaine J, Piraud M, Poussaint T, Prabhu SP, Reitman Z, Rodriguez A, Rudie JD, Shaikh IS, Shah LM, Sheth N, Shinohara RT, Tu W, Viswanathan K, Wang C, Ware JB, Wiestler B, Wiggins W, Anna Zapaishchykova, Aboian M, Bornhorst M, de Blank P, Deutsch M, Fouladi M, Hoffman L, Kann B, Lazow M, Mikael L, Nabavizadeh A, Packer R, Resnick A, Rood B, Vossough A, Bakas S, Linguraru MG. ArXiv. 2023 Jul 7:arXiv:2305.17033v2. Preprint. PMID: 37292481.
-
Novel Mechanisms and Future Opportunities for the Management of Radiation Necrosis in Patients Treated for Brain Metastases in the Era of Immunotherapy. Vaios EJ, Winter SF, Shih HA, Dietrich J, Peters KB, Floyd SR, Kirkpatrick JP, Reitman ZJ. Cancers (Basel). 2023 Apr 24;15(9):2432. doi: 10.3390/cancers15092432. PMID: 37173897.
-
Clinical Factors Associated With 30-Day Mortality Among Patients Undergoing Radiation Therapy for Brain Metastases. Natesan D, Carpenter DJ, Giles W, Oyekunle T, Niedzwiecki D, Reitman ZJ, Kirkpatrick JP, Floyd SR. Adv Radiat Oncol. 2023 Mar 8;8(4):101211. doi: 10.1016/j.adro.2023.101211. eCollection 2023 Jul-Aug. PMID: 37152484.
-
Ganglioglioma deep transcriptomics reveals primitive neuroectoderm neural precursor-like population. Regal JA, Guerra García ME, Jain V, Chandramohan V, Ashley DM, Gregory SG, Thompson EM, López GY, Reitman ZJ. Acta Neuropathol Commun. 2023 Mar 25;11(1):50. doi: 10.1186/s40478-023-01548-3. PMID: 36966348.
-
Inducing primary brainstem gliomas in genetically engineered mice using RCAS/TVA retroviruses and Cre/loxP recombination. Weidenhammer LB, Liu HQ, Luo L, Williams NT, Deland K, Kirsch DG, Reitman ZJ. STAR Protoc. 2023 Mar 17;4(1):102094. doi: 10.1016/j.xpro.2023.102094. Epub 2023 Feb 13. PMID: 36853662.
-
Outcomes in Patients with Intact and Resected Brain Metastasis Treated with 5-Fraction Stereotactic Radiosurgery. Carpenter DJ, Fairchild AT, Adamson JD, Fecci PE, Sampson JH, Herndon JE, Torok JA, Mullikin TC, Kim GJ, Reitman ZJ, Kirkpatrick JP, Floyd SR. Adv Radiat Oncol. 2022 Dec 29;8(2):101166. doi: 10.1016/j.adro.2022.101166. eCollection 2023 Mar-Apr. PMID: 36845614.
-
Prognostic Model for Intracranial Progression after Stereotactic Radiosurgery: A Multicenter Validation Study. Carpenter DJ, Natarajan B, Arshad M, Natesan D, Schultz O, Moravan MJ, Read C, Lafata KJ, Giles W, Fecci P, Mullikin TC, Reitman ZJ, Kirkpatrick JP, Floyd SR, Chmura SJ, Hong JC, Salama JK. Cancers (Basel). 2022 Oct 22;14(21):5186. doi: 10.3390/cancers14215186. PMID: 36358606.
-
The Effect of Atm Loss on Radiosensitivity of a Primary Mouse Model of Pten-Deleted Brainstem Glioma. Stewart CE, Guerra-García ME, Luo L, Williams NT, Ma Y, Regal JA, Ghosh D, Sansone P, Oldham M, Deland K, Becher OJ, Kirsch DG, Reitman ZJ. Cancers (Basel). 2022 Sep 17;14(18):4506. doi: 10.3390/cancers14184506. PMID: 36139666.
-
Epigenetic STING silencing is developmentally conserved in gliomas and can be rescued by methyltransferase inhibition. Low JT, Chandramohan V, Bowie ML, Brown MC, Waitkus MS, Briley A, Stevenson K, Fuller R, Reitman ZJ, Muscat AM, Hariharan S, Hostettler J, Danehower S, Baker A, Khasraw M, Wong NC, Gregory S, Nair SK, Heimberger A, Gromeier M, Bigner DD, Ashley DM. Cancer Cell. 2022 May 9;40(5):439-440. doi: 10.1016/j.ccell.2022.04.009. Epub 2022 Apr 28. PMID: 35487217. No abstract available.
-
A Need for More Molecular Profiling in Brain Metastases. Shen E, Van Swearingen AED, Price MJ, Bulsara K, Verhaak RGW, Baëta C, Painter BD, Reitman ZJ, Salama AKS, Clarke JM, Anders CK, Fecci PE, Goodwin CR, Walsh KM. Front Oncol. 2022 Jan 25;11:785064. doi: 10.3389/fonc.2021.785064. eCollection 2021. PMID: 35145903.
-
PPM1D mutations are oncogenic drivers of de novo diffuse midline glioma formation. Khadka P, Reitman ZJ, Lu S, Buchan G, Gionet G, Dubois F, Carvalho DM, Shih J, Zhang S, Greenwald NF, Zack T, Shapira O, Pelton K, Hartley R, Bear H, Georgis Y, Jarmale S, Melanson R, Bonanno K, Schoolcraft K, Miller PG, Condurat AL, Gonzalez EM, Qian K, Morin E, Langhnoja J, Lupien LE, Rendo V, Digiacomo J, Wang D, Zhou K, Kumbhani R, Guerra Garcia ME, Sinai CE, Becker S, Schneider R, Vogelzang J, Krug K, Goodale A, Abid T, Kalani Z, Piccioni F, Beroukhim R, Persky NS, Root DE, Carcaboso AM, Ebert BL, Fuller C, Babur O, Kieran MW, Jones C, Keshishian H, Ligon KL, Carr SA, Phoenix TN, Bandopadhayay P. Nat Commun. 2022 Feb 1;13(1):604. doi: 10.1038/s41467-022-28198-8. PMID: 35105861.
-
Targeting the ATM Kinase to Enhance the Efficacy of Radiotherapy and Outcomes for Cancer Patients. García MEG, Kirsch DG, Reitman ZJ. Semin Radiat Oncol. 2022 Jan;32(1):3-14. doi: 10.1016/j.semradonc.2021.09.008. PMID: 34861994.
-
Radiosensitizing the Vasculature of Primary Brainstem Gliomas Fails to Improve Tumor Response to Radiation Therapy. Deland K, Mercer JS, Crabtree DM, Guerra Garcia ME, Reinsvold M, Campos LDS, Williams NT, Luo L, Ma Y, Reitman ZJ, Becher OJ, Kirsch DG. Int J Radiat Oncol Biol Phys. 2022 Mar 1;112(3):771-779. doi: 10.1016/j.ijrobp.2021.09.047. Epub 2021 Oct 5. PMID: 34619331.
-
A Modified Nucleoside 6-Thio-2'-Deoxyguanosine Exhibits Antitumor Activity in Gliomas. Yu S, Wei S, Savani M, Lin X, Du K, Mender I, Siteni S, Vasilopoulos T, Reitman ZJ, Ku Y, Wu D, Liu H, Tian M, Chen Y, Labrie M, Charbonneau CM, Sugarman E, Bowie M, Hariharan S, Waitkus M, Jiang W, McLendon RE, Pan E, Khasraw M, Walsh KM, Lu Y, Herlyn M, Mills G, Herbig U, Wei Z, Keir ST, Flaherty K, Liu L, Wu K, Shay JW, Abdullah K, Zhang G, Ashley DM. Clin Cancer Res. 2021 Dec 15;27(24):6800-6814. doi: 10.1158/1078-0432.CCR-21-0374. Epub 2021 Sep 30. PMID: 34593527.
-
Mitogenic and progenitor gene programmes in single pilocytic astrocytoma cells. Reitman ZJ, Paolella BR, Bergthold G, Pelton K, Becker S, Jones R, Sinai CE, Malkin H, Huang Y, Grimmet L, Herbert ZT, Sun Y, Weatherbee JL, Alberta JA, Daley JF, Rozenblatt-Rosen O, Condurat AL, Qian K, Khadka P, Segal RA, Haas-Kogan D, Filbin MG, Suva ML, Regev A, Stiles CD, Kieran MW, Goumnerova L, Ligon KL, Shalek AK, Bandopadhayay P, Beroukhim R. Nat Commun. 2019 Aug 19;10(1):3731. doi: 10.1038/s41467-019-11493-2. PMID: 31427603.
-
New Directions in the Treatment of Glioblastoma. Reitman ZJ, Winkler F, Elia AEH. Semin Neurol. 2018 Feb;38(1):50-61. doi: 10.1055/s-0038-1623534. Epub 2018 Mar 16. PMID: 29548052.
-
Impact of pemetrexed on intracranial disease control and radiation necrosis in patients with brain metastases from non-small cell lung cancer receiving stereotactic radiation. Cagney DN, Martin AM, Catalano PJ, Reitman ZJ, Mezochow GA, Lee EQ, Wen PY, Weiss SE, Brown PD, Ahluwalia MS, Arvold ND, Tanguturi SK, Haas-Kogan DA, Alexander BM, Redig AJ, Aizer AA. Radiother Oncol. 2018 Mar;126(3):511-518. doi: 10.1016/j.radonc.2018.01.005. Epub 2018 Feb 3. PMID: 29398153.
-
Synthesis and evaluation of radiolabeled AGI-5198 analogues as candidate radiotracers for imaging mutant IDH1 expression in tumors. Chitneni SK, Reitman ZJ, Spicehandler R, Gooden DM, Yan H, Zalutsky MR. Bioorg Med Chem Lett. 2018 Feb 15;28(4):694-699. doi: 10.1016/j.bmcl.2018.01.015. Epub 2018 Jan 12. PMID: 29366652.
-
The genome-wide mutational landscape of pituitary adenomas. Song ZJ, Reitman ZJ, Ma ZY, Chen JH, Zhang QL, Shou XF, Huang CX, Wang YF, Li SQ, Mao Y, Zhou LF, Lian BF, Yan H, Shi YY, Zhao Y. Cell Res. 2016 Nov;26(11):1255-1259. doi: 10.1038/cr.2016.114. Epub 2016 Sep 27. PMID: 27670697. No abstract available.
-
Radiolabeled inhibitors as probes for imaging mutant IDH1 expression in gliomas: Synthesis and preliminary evaluation of labeled butyl-phenyl sulfonamide analogs. Chitneni SK, Reitman ZJ, Gooden DM, Yan H, Zalutsky MR. Eur J Med Chem. 2016 Aug 25;119:218-30. doi: 10.1016/j.ejmech.2016.04.066. Epub 2016 Apr 28. PMID: 27163884.
-
Detecting somatic mutations in genomic sequences by means of Kolmogorov-Arnold analysis. Gurzadyan VG, Yan H, Vlahovic G, Kashin A, Killela P, Reitman Z, Sargsyan S, Yegorian G, Milledge G, Vlahovic B. R Soc Open Sci. 2015 Aug 26;2(8):150143. doi: 10.1098/rsos.150143. eCollection 2015 Aug. PMID: 26361546
-
Smaller protein, larger therapeutic potential: PPM1D as a new therapeutic target in brainstem glioma. Reitman ZJ. Pharmacogenomics. 2014 Sep;15(13):1639-41. doi: 10.2217/pgs.14.123. PMID: 25410889. No abstract available.
-
Genetic dissection of leukemia-associated IDH1 and IDH2 mutants and D-2-hydroxyglutarate in Drosophila. Reitman ZJ, Sinenko SA, Spana EP, Yan H. Blood. 2015 Jan 8;125(2):336-45. doi: 10.1182/blood-2014-05-577940. Epub 2014 Nov 14. PMID: 25398939.
-
Cancer-associated isocitrate dehydrogenase 1 (IDH1) R132H mutation and d-2-hydroxyglutarate stimulate glutamine metabolism under hypoxia. Reitman ZJ, Duncan CG, Poteet E, Winters A, Yan LJ, Gooden DM, Spasojevic I, Boros LG, Yang SH, Yan H. J Biol Chem. 2014 Aug 22;289(34):23318-28. doi: 10.1074/jbc.M114.575183. Epub 2014 Jul 1. PMID: 24986863.
-
Exome sequencing identifies somatic gain-of-function PPM1D mutations in brainstem gliomas. Zhang L, Chen LH, Wan H, Yang R, Wang Z, Feng J, Yang S, Jones S, Wang S, Zhou W, Zhu H, Killela PJ, Zhang J, Wu Z, Li G, Hao S, Wang Y, Webb JB, Friedman HS, Friedman AH, McLendon RE, He Y, Reitman ZJ, Bigner DD, Yan H. Nat Genet. 2014 Jul;46(7):726-30. doi: 10.1038/ng.2995. Epub 2014 Jun 1. PMID: 24880341.
-
Mutations in IDH1, IDH2, and in the TERT promoter define clinically distinct subgroups of adult malignant gliomas. Killela PJ, Pirozzi CJ, Healy P, Reitman ZJ, Lipp E, Rasheed BA, Yang R, Diplas BH, Wang Z, Greer PK, Zhu H, Wang CY, Carpenter AB, Friedman H, Friedman AH, Keir ST, He J, He Y, McLendon RE, Herndon JE 2nd, Yan H, Bigner DD. Oncotarget. 2014 Mar 30;5(6):1515-25. doi: 10.18632/oncotarget.1765. PMID: 24722048.
-
Promoting a new brain tumor mutation: TERT promoter mutations in CNS tumors. Reitman ZJ, Pirozzi CJ, Yan H. Acta Neuropathol. 2013 Dec;126(6):789-92. doi: 10.1007/s00401-013-1207-5. PMID: 24217890. No abstract available.
-
The genetic landscape of anaplastic astrocytoma. Killela PJ, Pirozzi CJ, Reitman ZJ, Jones S, Rasheed BA, Lipp E, Friedman H, Friedman AH, He Y, McLendon RE, Bigner DD, Yan H. Oncotarget. 2014 Mar 30;5(6):1452-7. doi: 10.18632/oncotarget.1505. PMID: 24140581.
-
Relationship Between Time of First Publication and Subsequent Publication Success Among Non-PhD Physician-Scientists. Riggs KR, Reitman ZJ, Mielenz TJ, Goodman PC. J Grad Med Educ. 2012 Jun;4(2):196-201. doi: 10.4300/JGME-D-11-00068.1. PMID: 23730441.
-
Releasing the block: setting differentiation free with mutant IDH inhibitors. Pirozzi CJ, Reitman ZJ, Yan H. Cancer Cell. 2013 May 13;23(5):570-2. doi: 10.1016/j.ccr.2013.04.024. PMID: 23680144.
-
Exomic sequencing of four rare central nervous system tumor types. Bettegowda C, Agrawal N, Jiao Y, Wang Y, Wood LD, Rodriguez FJ, Hruban RH, Gallia GL, Binder ZA, Riggins CJ, Salmasi V, Riggins GJ, Reitman ZJ, Rasheed A, Keir S, Shinjo S, Marie S, McLendon R, Jallo G, Vogelstein B, Bigner D, Yan H, Kinzler KW, Papadopoulos N. Oncotarget. 2013 Apr;4(4):572-83. doi: 10.18632/oncotarget.964. PMID: 23592488.
-
TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He TC, He Y, Hruban RH, Jallo GI, Mandahl N, Meeker AK, Mertens F, Netto GJ, Rasheed BA, Riggins GJ, Rosenquist TA, Schiffman M, Shih IeM, Theodorescu D, Torbenson MS, Velculescu VE, Wang TL, Wentzensen N, Wood LD, Zhang M, McLendon RE, Bigner DD, Kinzler KW, Vogelstein B, Papadopoulos N, Yan H. Proc Natl Acad Sci U S A. 2013 Apr 9;110(15):6021-6. doi: 10.1073/pnas.1303607110. Epub 2013 Mar 25. PMID: 23530248.
-
Disruption of wild-type IDH1 suppresses D-2-hydroxyglutarate production in IDH1-mutated gliomas. Jin G, Reitman ZJ, Duncan CG, Spasojevic I, Gooden DM, Rasheed BA, Yang R, Lopez GY, He Y, McLendon RE, Bigner DD, Yan H. Cancer Res. 2013 Jan 15;73(2):496-501. doi: 10.1158/0008-5472.CAN-12-2852. Epub 2012 Nov 30. PMID: 23204232.
-
Enzyme redesign guided by cancer-derived IDH1 mutations. Reitman ZJ, Choi BD, Spasojevic I, Bigner DD, Sampson JH, Yan H. Nat Chem Biol. 2012 Nov;8(11):887-9. doi: 10.1038/nchembio.1065. Epub 2012 Sep 23. PMID: 23001033.
-
Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, Rodriguez FJ, Rosemberg S, Oba-Shinjo SM, Nagahashi Marie SK, Bettegowda C, Agrawal N, Lipp E, Pirozzi C, Lopez G, He Y, Friedman H, Friedman AH, Riggins GJ, Holdhoff M, Burger P, McLendon R, Bigner DD, Vogelstein B, Meeker AK, Kinzler KW, Papadopoulos N, Diaz LA, Yan H. Oncotarget. 2012 Jul;3(7):709-22. doi: 10.18632/oncotarget.588. PMID: 22869205.
-
2-hydroxyglutarate production, but not dominant negative function, is conferred by glioma-derived NADP-dependent isocitrate dehydrogenase mutations. Jin G, Reitman ZJ, Spasojevic I, Batinic-Haberle I, Yang J, Schmidt-Kittler O, Bigner DD, Yan H. PLoS One. 2011 Feb 4;6(2):e16812. doi: 10.1371/journal.pone.0016812. PMID: 21326614.
-
Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Reitman ZJ, Jin G, Karoly ED, Spasojevic I, Yang J, Kinzler KW, He Y, Bigner DD, Vogelstein B, Yan H. Proc Natl Acad Sci U S A. 2011 Feb 22;108(8):3270-5. doi: 10.1073/pnas.1019393108. Epub 2011 Feb 2. PMID: 21289278
-
IDH1(R132) mutation identified in one human melanoma metastasis, but not correlated with metastases to the brain. Lopez GY, Reitman ZJ, Solomon D, Waldman T, Bigner DD, McLendon RE, Rosenberg SA, Samuels Y, Yan H. Biochem Biophys Res Commun. 2010 Jul 30;398(3):585-7. doi: 10.1016/j.bbrc.2010.06.125. Epub 2010 Jul 13. PMID: 20603105.
-
Isocitrate dehydrogenase 1 and 2 mutations in cancer: alterations at a crossroads of cellular metabolism. Reitman ZJ, Yan H. J Natl Cancer Inst. 2010 Jul 7;102(13):932-41. doi: 10.1093/jnci/djq187. Epub 2010 May 31. PMID: 20513808.
-
IDH1 and IDH2: not your typical oncogenes. Reitman ZJ, Parsons DW, Yan H. Cancer Cell. 2010 Mar 16;17(3):215-6. doi: 10.1016/j.ccr.2010.02.024. PMID: 20227034.
-
IDH1 and IDH2 hotspot mutations are not found in canine glioma. Reitman ZJ, Olby NJ, Mariani CL, Thomas R, Breen M, Bigner DD, McLendon RE, Yan H. Int J Cancer. 2010 Jul 1;127(1):245-6. doi: 10.1002/ijc.25017. PMID: 19877121.
-
Picornavirus genome replication. Identification of the surface of the poliovirus (PV) 3C dimer that interacts with PV 3Dpol during VPg uridylylation and construction of a structural model for the PV 3C2-3Dpol complex. Shen M, Reitman ZJ, Zhao Y, Moustafa I, Wang Q, Arnold JJ, Pathak HB, Cameron CE. J Biol Chem. 2008 Jan 11;283(2):875-88. doi: 10.1074/jbc.M707907200. Epub 2007 Nov 9. PMID: 17993457.
People
Lab Member Bios
Zach Reitman, MD, PhD
Zach Reitman is a physician-scientist at Duke. As a physician, he provides radiation oncology care for patients with brain and spine tumors at the Duke Cancer Institute. As a scientist, he and his team work to understand the molecular genetics of childhood and adult brain tumors in order to develop new treatment strategies that are more effective and have fewer side effects. Zach completed his MD and PhD training at Duke where he studied brain tumor genetics in the laboratory of Professor Hai Yan, a world expert in genomic analyses of brain tumors. He completed radiation oncology residency training at the Harvard Radiation Oncology Program, where he completed a research fellowship at the Dana-Farber Cancer Institute and the Broad Institute in the laboratories of two brain tumor genomic research experts, Dr. Rameen Beroukhim and Dr. Pratiti Bandopadhayay. Zach and his team are now leveraging genomic analyses and mouse modeling techniques to carry out new lines of investigation in the Reitman Lab. In his free time, Zach enjoys hiking, biking and spending time with his family.
Bronwen Foreman
Bronwen Foreman is a third-year medical student and recipient of the 2020 Rauch Family Leaders Scholarship at Duke. She first became interested in oncology research while working at Massachusetts General Hospital, where she served as the primary research coordinator on a trial investigating the combination of immunotherapy and proton radiation in the treatment of pancreatic and colorectal cancer. She began working with Dr. Reitman in 2020 on a clinical research project involving the use of stereotactic radiosurgery in adult primary central nervous system lymphoma. She then joined the basic science lab in 2022 and is currently working on a project identifying mechanisms of treatment resistance in diffuse intrinsic pontine gliomas. Bronwen will be applying to Medicine-Pediatrics for residency and is interested in transitional oncologic care for adult patients with a history of pediatric brain tumors. Outside of medicine, she enjoys baking, hiking and reading classic literature.
Abby Groth
Abby Groth is an undergraduate research assistant and joined the Reitman Lab in the spring of 2023. Originally from Wisconsin, Abby is majoring in biology and psychology with a medical sociology minor and plans to graduate in May 2024. She has always been interested in cancer research and is interested in pursing an MD/PhD and working in pediatric oncology. Outside of the lab, Abby enjoys strength training, hiking, cooking and rooting for Duke athletics.
Harrison Liu
Harrison Liu joined the Reitman Lab in the summer of 2022. He graduated in 2021 from the University of North Carolina at Chapel Hill with a Bachelor of Science in biology. Harrison is fascinated by oncology research and looks forward to continuing it in medical or graduate school. No matter where he ends up, Harrison is determined to drive scientific discovery and innovation in his career to better patient outcomes. Besides science, Harrison enjoys cooking, playing golf and cheering on the best college basketball team – his alma mater.
Spencer Maingi
Spencer joined the Reitman Lab in the summer of 2023. Prior to joining the lab, he worked at Locus Biosciences as a research technician intern and as a CNA at Duke Regional Hospital. He graduated from UNC-Chapel Hill in 2021 with a BS in biochemistry and neuroscience. Spencer has had a long-standing interest in brain cancer, motivated in large part by his previous experiences with researcher Dr. Shawn Hingtgen and neuro-oncologist Dr. Simon Khagi. In the future, he plans to attend medical school and continue doing brain tumor research to help improve patient lives at the bench and bedside. Outside of work, Spencer loves to cook, spend time with his family and cheer on Penn State football and UNC basketball.
Avneesh Saravanapavan
Avneesh Saravanapavan is a passionate individual on a journey towards a career in medicine, fueled by a keen interest in pursuing an MD/PhD in biology. His fascination extends beyond the realms of traditional medicine, as he actively engages in statistical analysis within the medical field. When not immersed in the demanding pre-med requirements, Avneesh finds solace in the pages of books and explores eateries in search of novel culinary experiences.
Josh Tolliver
Josh joined the Reitman Lab from the MGM department at Duke University in the Summer of 2023. He graduated from the University of North Carolina at Chapel Hill in May 2022 with a BS in biology and a BA in Asian studies – Chinese concentration. Though he has experienced multiple fields of biological research in his career so far, Josh has always had a longstanding interest in cancer biology in particular and is excited to learn more about this field in the Reitman Lab. His longterm plans are to attend graduate school to earn his PhD in biology; he is eager to contribute to scientific discovery and innovation. Outside of science, Josh enjoys playing guitar, spending time outside, cooking and reading.
Vennesa Valentine
Vennesa Valentine has a strong passion for making a positive mark in the field of pharmacology and within her academic and local community. She actively participates in mentorship and community service, both at Duke University and as a Passport to College Peer Mentor. In her academic pursuit, Vennesa demonstrates a keen interest in cancer research, where she seeks to make valuable contributions to the advancement of cancer therapeutics and treatments. In her leisure time, Vennesa finds joy in experiencing cultures and savoring new culinary experiences.
Nerissa Williams
Nerissa Williams joined the Reitman Lab in the spring of 2023. Prior to that, she was with David Kirsch’s lab as a Lab Research Analyst I and worked on various experiments including a joint project with NASA at Brookhaven National Laboratory in Upton, NY. She is currently the main operator for the small animal irradiator, which images and treats genetically engineered mice using a novel micro-CT/micro-irradiator research platform for the Reitman Lab as well as other labs at Duke University. Nerissa graduated from NC State University with a BS in animal science. Outside of work, she has competed as an amateur USA boxer and now enjoys coaching. She also enjoys traveling, cooking and DJing.
Sophie Wu
Sophie Wu is an undergraduate research assistant in the Reitman Lab at Duke University from Seattle, WA. She is pre-health student working towards a bachelor’s degree in biomedical engineering and is set to graduate in May 2024. In the Reitman Lab, Sophie is developing and testing the RCAS-TVA-CRISPR-Cas9 system for future precision tumor modeling. After graduation, she hopes to attend medical school, potentially as an aspiring physician scientist. During her free time, Sophie enjoys playing tennis, drawing and spending time with friends and family.
Lab Alumni
Lab Alumni Bios
Debosir Ghosh
Debosir Ghosh was an undergraduate research assistant when he joined the Reitman Lab. He is currently pursuing degrees in neuroscience and chemistry and also working towards a minor in piano performance. Since high school, Debosir has been especially captivated by radiation oncology and how the findings of genetically-engineered mouse-models can be used to potentially treat cancer patients. In the lab, he was working with Dr. Reitman to investigate brainstem gliomas and aspires to eventually become a physician. In addition to his studies in science and music, Debosir is also an avid aviation enthusiast and enjoys plane spotting with friends and family.
Maria Guerra Garcia, BS
Maria Guerra Garcia studied biomedical engineering and biology at the University of Texas at San Antonio and completed an honors thesis on the development of an animal model of Leptomeningeal Carcinomatosis to examine the efficacy of Rhenium-186 Nanoliposomes and other therapeutics. She was born and raised in Monterrey, Mexico, and is especially passionate about the fight against pediatric cancer and mental health advocacy. During her free time, she enjoys practicing piano, playing with her cat Daisy-Lu and volunteering as a crisis counselor for the Crisis Text Line. In the Reitman Lab, Maria investigated different brainstem glioma genotypes that are radiosensitized by the deletion of the ATM gene with the hope of increasing the therapeutic window of radiation and improving survival rates for adults and children with brainstem tumors. She aspires to become a physician scientist focused on caring for cancer patients, conducting research to improve treatments and outcomes, and advocating for her patients through non-profit organizational work. Maria is now a PhD student in biomedical engineering at Washington University in St. Louis.
Joshua A. Regal, MD, PhD
Joshua Regal was a postdoctoral associate in radiation oncology at Duke when he joined the Reitman Lab. Joshua completed his MD and PhD training at the University of Michigan. His PhD work with Dr. David Ferguson involved deciphering roles of dysfunction of the DNA damage response/repair factor MRE11 in carcinogenesis and human genetic disease pathogenesis. As a postdoctoral fellow at University of British Columbia with Dr. Phil Hieter, his work involved mechanistically dissecting cohesin genetic interactions to better understand the consequences of cancer-associated cohesin alterations with potential implications for cancer diagnosis, prognostication and treatment. He joined the Reitman group to better understand brain tumors on a cellular and molecular level, which could potentially be leveraged for better understanding of tumor development and maintenance and therapeutic management and development. After completing his postdoctoral work in the lab, Dr. Regal began a position as Assistant Professor of Radiation Oncology at Roswell Park Cancer Institute in Buffalo, NY.
Connor Stewart, BS
Connor Stewart was a research technician in the Reitman Lab at Duke. He obtained his bachelor’s degree in biological sciences from North Carolina State University in 2020, with a focus on cell and molecular biology. As a biologist, Connor is interested in studying the molecular mechanisms that underlie cancer and cancer treatment. He worked with Dr. Reitman to investigate the genetics of brain tumors, with the goal of improving treatment options and clinical outcomes. Connor hopes to eventually earn a PhD in cancer biology and to conduct research that helps improve our understanding of cancer. In his spare time, Connor enjoys watching baseball and playing the violin and guitar. Connor is now a PhD student in cancer biology at Emory University.
Kevin Tu
Kevin Tu was an undergraduate at the University of Maryland who joined the Reitman Lab as an Amgen Scholar. Working towards a double degree in biology and economics, Kevin is interested in uncovering and targeting the genetics that drives cancer progression and onset to improve patient outcomes. An aspiring physician-scientist, Kevin hopes to earn an MD-PhD in cancer biology and develop gene-based therapies, diagnostic tools and biomarkers for aggressive and treatment-resistant cancers. In his spare time, he enjoys sketching with charcoal and playing video games with friends. He is now pursuing a prestigious Churchill Scholarship at Cambridge University in the UK before matriculating into medical school.
Loren Weidenhammer, BS
Loren Weidenhammer was a research technician in the Reitman Lab at Duke. She received a Bachelor of Science in biology with a double major in history from the University of North Carolina at Chapel Hill. As a biologist, Loren is interested in studying the underlying molecular mechanisms of cancer and how those mechanisms can be applied to improving cancer radiation treatment. She worked with Dr. Reitman to investigate the molecular genetics of brain cancer tumors that are found in children and adults, with the goal of improving cancer treatment and clinical outcomes for patients. In addition, she worked to identify and target the key molecular players in the pathways that promote brain tumor formation. Loren is currently earning a PhD in the prestigious Duke Pathology program with plans to become a research professor. In her spare time, Loren likes to travel, read and play with her pets.
News
LaBella and Duke Faculty Among Authors of the 2024 BraTS-MEN-RT Dataset
Reitman Awarded ALSF Grant
FLASH Forward: Duke Researchers Explore Ultra-High Dose Rate FLASH Radiation Therapy for Brain Tumors
Vaios, Floyd, Reitman To Collaborate on Pilot Study
Reitman Lab Awarded 2024 DCI Grant
Reitman Awarded K08, New Investigator Award
Reitman Awarded Fellow Grant from The St. Baldrick’s Foundation
Team Member Spotlight: Zach Reitman, MD, PhD
Contact
Email Dr. Reitman at zjr@duke.edu.





















