Kyle Lafata, PhD, received a new grant from the Duke Cancer Institute titled "Dissecting the role of immune cell topology and spatial gene expression in HPV+ OPC racial disparities." The project is in partnership with Tammara Watts, MD, PhD, of Head and Neck Surgery and Communication Sciences. Drs. Lafata and Watts will integrate computational tissue phenotyping (Lafata Lab) with spatial biology techniques (Watts Lab) to study the tumor microenvironment of oropharyngeal head and neck cancer.
These tumors are highly heterogeneous with respect to gene expression and therapeutic response and represent the third worst mortality gap between Black and White patients in the United States. For example, overall survival of Black patients diagnosed with oropharyngeal cancer is significantly worse than White patients regardless of socioeconomic status, which strongly suggests biologic variables contribute to outcomes. Moreover, the globally poor response to immunotherapy seen in all patients with head and neck cancer suggest there are negative immunoregulatory mechanisms within the tumor microenvironment that inhibit immune response, which may also be potential drivers of racial differences in HPV-mediated etiology and outcome.
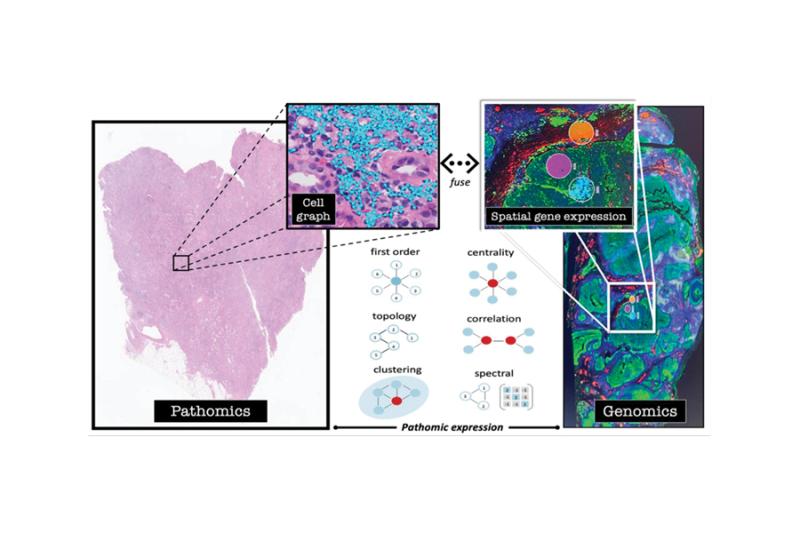
Drs. Lafata and Watts hope to discover tumor promoting pathways, negative immunoregulatory mechanisms and/or pattern preserving features of the tumor microenvironment that link gene expression to spatial interaction among immune cells. First, mathematical graph features will determine whether immune cell topology of the tumor microenvironment is a driver of racial disparity in HPV-mediated oropharyngeal cancer. These cell graphs will then be computationally fused with spatial gene expression to identify molecular drivers of cellular topology and potential cross-talk between the tumor genotype and phenotype. These hypothesis generating data will necessitate further study as a biologic mechanism(s) underlying decreased survival observed for Black patients with oropharyngeal cancer.
Photo: Fusion of mathematical immune cell topology with spatial gene expression. Drs. Lafata and Watts plan to combine digital pathology and pathomics with Nanostring technology to search for new biology linking immune cell topology to spatial gene expression. When integrated, this approach facilitates simultaneous phenotyping and genotyping of the tumor microenvironment.