

In the lab of scientist Kyle Lafata, PhD, research isn’t confined to a single discipline – it spans high-performance scientific computing, experimental radiation biology and applied mathematics, forming an interdisciplinary approach where each part informs and strengthens the others.
“We do a little bit of everything,” said Dr. Lafata, the Thaddeus V. Samulski Associate Professor of Radiation Oncology. “I think what makes the lab unique is the different areas of science we stitch together. We use many different mathematical and computational techniques – differential equations, stochastic processes, deep learning, agent-based models – to interrogate tumor dynamics, immune responses and radiation effects. At the same time, we conduct complementary experiments using systems biology approaches – mouse models, advanced imaging, digital pathology, single cell multiplex sequencing – to investigate the biological mechanisms driving radiation resistance.”
This integrated approach is central to Dr. Lafata’s research program, which is federally funded on multiple grants from the National Institutes of Health and the Department of Defense. His three medical physics PhD students exemplify the lab’s strategic commitment to multidisciplinary science. Casey Heirman tends towards the experimental – as Dr. Lafata explains it, “she’s at the bench, working with mice, analyzing data – she’s truly hands-on.” At the other end of the spectrum is Jack Stevens, who has a physics background and works with simulations, exploring tumor growth modeling and response to radiation using applied mathematics. Bridging the experimental and theoretical is Breylon Riley, who works with advanced computational algorithms and the data science side of things.
For Dr. Lafata, the strength of this integrated lab model lies in the interplay between data-driven and mechanistic approaches. “While machine learning and other purely data-driven methods are highly effective at uncovering complex patterns, they typically do not capture underlying biological or physical mechanisms,” he explained. “On the other hand, theoretical models offer mechanistic insight but are limited by oversimplification and ill-conditioned solutions. Hybrid models offer the best of both worlds by combining the mechanistic insight of theoretical frameworks with the flexibility of data-driven methods.” The emerging field of physics-informed machine learning embeds physical laws into neural networks to improve generalization and ensure physically constrained predictions.
“Merging physics-informed machine learning with experimental cancer biology data creates a synergistic framework that leverages both real-world evidence and mechanistic understanding,” he said.
Beyond the Bench: Computational Radiation Biology

“I’ve always loved medicine and anatomy,” said Casey Heirman, “but in college, physics caught me by surprise.” As an undergraduate student, Casey chose to study both biology and physics, an unconventional combination that ended up defining her future. “I didn’t even know medical physics was a field,” she said. “But once I discovered it, it felt like everything I was interested in – everything I was good at – came together in one place.” That realization led her to the Duke Medical Physics Graduate Program, and to the Lafata lab. “So many medical physics labs are very physics-heavy,” said Casey. “I wanted to stay connected to biology – to the wet lab, to the exciting biological questions, so when I read that Dr. Lafata’s work involved mouse models, I got really excited.”
“When I joined the lab, I remember saying to Dr. Lafata, ‘I really want to be part of the wet-lab side of our mission; is there any way I can work with the researchers doing mouse experiments?’” Dr. Lafata liked the idea and set Casey up with collaborating physician-scientists Yvonne Mowery, MD, PhD, and Tammara Watts, MD, PhD. “Dr. Lafata tailored the project to what I was excited about and introduced me to an incredible mentorship team, and that made all the difference,” said Casey. “He didn’t just let me bring my biology background to the lab – he made space for it and created projects around it. And I’ve seen him do the same thing with other students, too. That’s why our lab is so dynamic. We’re all working on different parts of the same problem, but from the angles that excite us most.”
Casey’s current research centers on using mouse models of head and neck cancer to explore whether imaging data – specifically PET/CT and digital pathology – can be analyzed using advanced computational algorithms to probe underlying biology and predict radiation response. “What I really like about working in the Lafata lab is that we all have our different niches. And we’re constantly in conversation; if my lab mates need to better understand our experimental results or want to brainstorm the biological rationale of their models, I can help. If I need help writing computer code, they’re only a cubicle away.”
Applied Mathematics: Simulating Dynamic Tumor Behavior

For Jack Stevens, theory became most compelling when it stopped being theoretical. At the University of St. Andrews in Scotland, he trained in mathematics and theoretical physics before deciding to pivot toward something more applied. “I really enjoyed the analytical side of physics, but I was searching for a more tangible application.” Medical physics – especially its overlap with radiology and oncology – offered that bridge.
Drawn to Duke’s reputation for combining a research-heavy experience with clinical problems, Jack eventually joined Dr. Lafata’s lab, where his work in applied mathematics is a critical piece of the lab’s current science. “I think what really stood out to me when I learned about Dr. Lafata’s work was the opportunity to use the tools I already had – differential equations, mathematical modeling – and apply them to challenges in cancer imaging and treatment.”
Jack’s current research sits at the intersection of quantitative imaging and simulation science. Using advanced mathematical tools like Fokker-Planck dynamics, he’s developing methods that help better understand how cancer changes on medical images to simulate tumor evolution during radiation therapy. “The first big project I worked on was tied to a clinical trial at Duke involving patients with oropharyngeal cancer,” he said. “These patients had imaging done before and during treatment, but we wanted to simulate what happened to patients in between the two scans.”

Generating these “in between” images allow researchers to extract radiomic features over time, potentially identifying new biomarkers that better predict how tumors will respond to therapy. Jack’s recent work extends this further, dividing tumors into spatial “habitats” that could one day help clinicians target treatment more precisely. “The ultimate hope is to make radiation therapy more personalized, and spare side effects of unnecessary dosing,” he said.
Jack is quick to emphasize how essential the interdisciplinary structure and collaboration of the Lafata lab is to his work. “Having people like Casey, who’s collecting this experimental data in real-time, and Breylon, who’s testing our biomarker robustness using computational techniques – it’s incredibly valuable, because both of them are invested in better understanding the underlying biological aspects of our work,” he said. “For example, I’ve used data from Casey’s animal model experiments to validate my simulations, and insights from Breylon’s sensitivity analyses have helped me account for uncertainties in imaging parameters.”
All this is to say, in Dr. Lafata’s lab, theory doesn’t exist in a vacuum – it’s part of the feedback loop where computation, experimentation and simulation all inform each other. “It’s rare to find a lab that spans this full spectrum,” said Jack. “But it’s exactly what makes our work more robust, relevant and hopefully, one day, clinically impactful.”
Data Integration: Decoding Multiscale Tumor Biology

When Breylon Riley first stumbled across the field of medical physics, it was via a Google search. “I literally just typed in ‘medicine’ and ‘physics’ and medical physics popped up,” he said with a laugh. That search led him from an applied physics undergraduate path at the University of California, Davis to the Duke Medical Physics Graduate Program – and to the Lafata lab, where he’s been working for the past five years. Breylon’s work stands at the intersection of imaging, pathology and transcriptomics, with a focus on head and neck squamous cell carcinoma. “Dr. Lafata recognized my interest in some of the non-traditional aspects of medical physics – not strictly radiation therapy, not strictly diagnostic imaging,” he said. “So, I guess you can say what drew me in to the Lafata lab initially was the idea of going into this uncharted territory.”
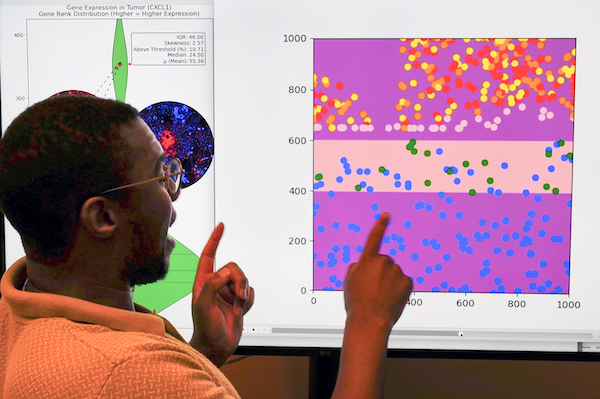
Breylon’s research explores multiscale phenotyping, or investigating tumors from the level of radiological imaging all the way down to gene expression. His early work involved extracting radiomic features from PET/CT scans to predict treatment outcomes, though that scope has since expanded. His current projects aim to understand how spatial cell architecture and underlying gene expression impact a tumor’s metabolic response to therapy – an effort that could help explain why immunotherapy has had limited success in improving survival rates for head and neck cancer patients, even as it proves successful with other cancer types. “We’re trying to figure out what’s going on in the tumor microenvironment and if we can use that knowledge, these measurements of gene expression, of cell topography, of tumor metabolism, to personalize treatment.”
Breylon occupies a kind of middle ground between Casey and Jack, drawing on both wet-lab data and computational analysis. “I like to think of myself as squarely between them,” he said. “Jack and I are constantly thinking about what’s hidden in the data that the human eye can’t see. And Casey’s work helps validate those ideas with real biological systems.” That collaborative spirit is what keeps the research both fresh and grounded. “You’re not just solving a problem in a vacuum,” said Breylon. “You’re building something together.”
Toward New Frontiers: Cancer’s Digital Revolution

After several years of foundational work in mathematical modeling, computational phenotyping and experimental cancer biology, the team is channeling these strengths into a unifying vision: digital twins of tumors. “We’ve started developing mechanistically informed and data-calibrated digital twins as virtual representations of tumors and their microenvironment,” said Dr. Lafata. “Grounded in applied mathematics and informed by imaging, pathology and molecular data from preclinical and clinical sources, these models can serve as a data-driven simulation framework for mechanistic research, drug screening and biomarker discovery.”
This work represents a natural progression of the lab’s multidisciplinary efforts and provides a promising framework to better study tumor behavior and therapeutic resistance.